1. Process architecture connects the goal of delivering a safe product with the art and science of designing a safe building.
Experts in process architecture are, first and foremost, registered (licensed) architects who have pledged to protect the health, safety and welfare (HSW) of the public. Think of that pledge as the process architect’s compass, and the cGMP facility as the environment in which they are experienced navigators.
In practice, this combination of architectural integrity and cGMP considerations means that process architects are especially qualified to advocate for the safety of two key groups:
Patient safety
Every detail inside a facility that could impact a drug product’s safety and purity needs the meticulous attention of a specialist team. It’s the process architect’s role to make sure that happens. From day one, they assess each design decision from the context of contamination control and patient safety, ensuring that no detail goes overlooked.
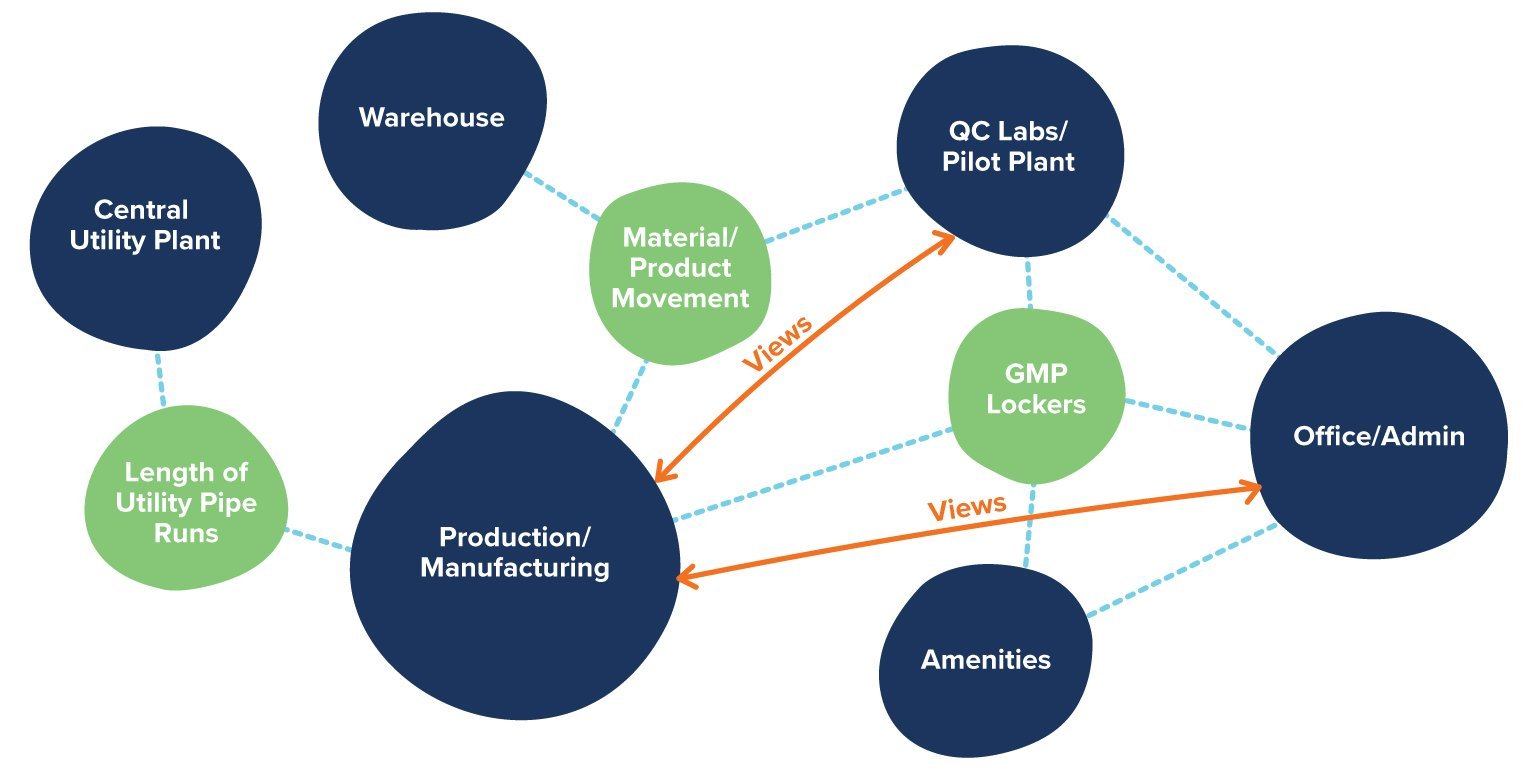
That means understanding and influencing the facility’s design from its highest levels (such as its room classifications, cGMP flows, and functional adjacencies) to its most granular details (such as the wall coatings inside a cleanroom, or the hardware for door interlock). Large or minute, complex or deceptively simple, every one of these details contributes to the safety of patients who rely on the facility’s output—and every one of them could benefit from the expert guidance of a process architect.
The safety of building occupants
Any environment in which potent materials, flammable solvents, and other hazardous chemicals are processed must contend with high-stakes health and safety considerations. This is especially true for manufacturers with antibody-drug conjugates (ADCs) in their pipeline, as well as those handling oligonucleotides, peptides, or the lipid nanoparticle (LNP) step in the mRNA manufacturing process.
Process architects have the specialized knowledge necessary to keep operators safe in these environments. They understand the stringent requirements in play when a space is classified as a hazardous occupancy (H-occupancy), and they can help project teams design appropriate fire-resistive assemblies, fire protection strategies, secondary containment, means of egress, safety stations, and more.
2. Process architecture supports your business case.
It takes a long history of diverse project work to shape a qualified process architect. For owners, that means a process architect draws from that extensive first-hand experience—the good and the lessons learned—to help deliver major business advantages.
Improved ROI on your capital spending
In order to get more bang for your capital bucks—that is, to maximize your use of every square foot in your facility—you need design solutions that elegantly balance both time and throughput considerations.
Process architects are especially good partners in this respect. They design your facility with the most efficient ratio of production targets versus available space possible, resulting in a highly utilized cGMP manufacturing operation.
Your brand, brought to life
A facility’s success from a business point of view isn’t only about the bottom-line. It’s also about how that facility establishes or elevates a company’s brand and reputation in the eyes of its stakeholders, its employees, and its end consumers. Here, too, process architects play a key role.
While thinking through the facility from a scientific and functional point of view, process architects are also considering the experience of being in that facility: What do you want employees, visitors, investors, and others to feel when they walk inside? What will your facility say about your priorities as a company—especially to the people who spend their workday between its walls?
Process architects achieve these brand-level goals through spatial design within the specific constraints—and opportunities—of a cGMP manufacturing facility. They know, for example, how and where to use glass walls to establish line-of-sight between manufacturing and administrative areas without impacting sensitive production areas, or which finishes are appropriate for maintaining cleanability while giving staff a pleasant experience of their environment.
These details can have a cascading effect on your facility’s business performance in terms of attracting and retaining top-performing staff, demonstrating success to investors, and building company-wide support for your overall mission.
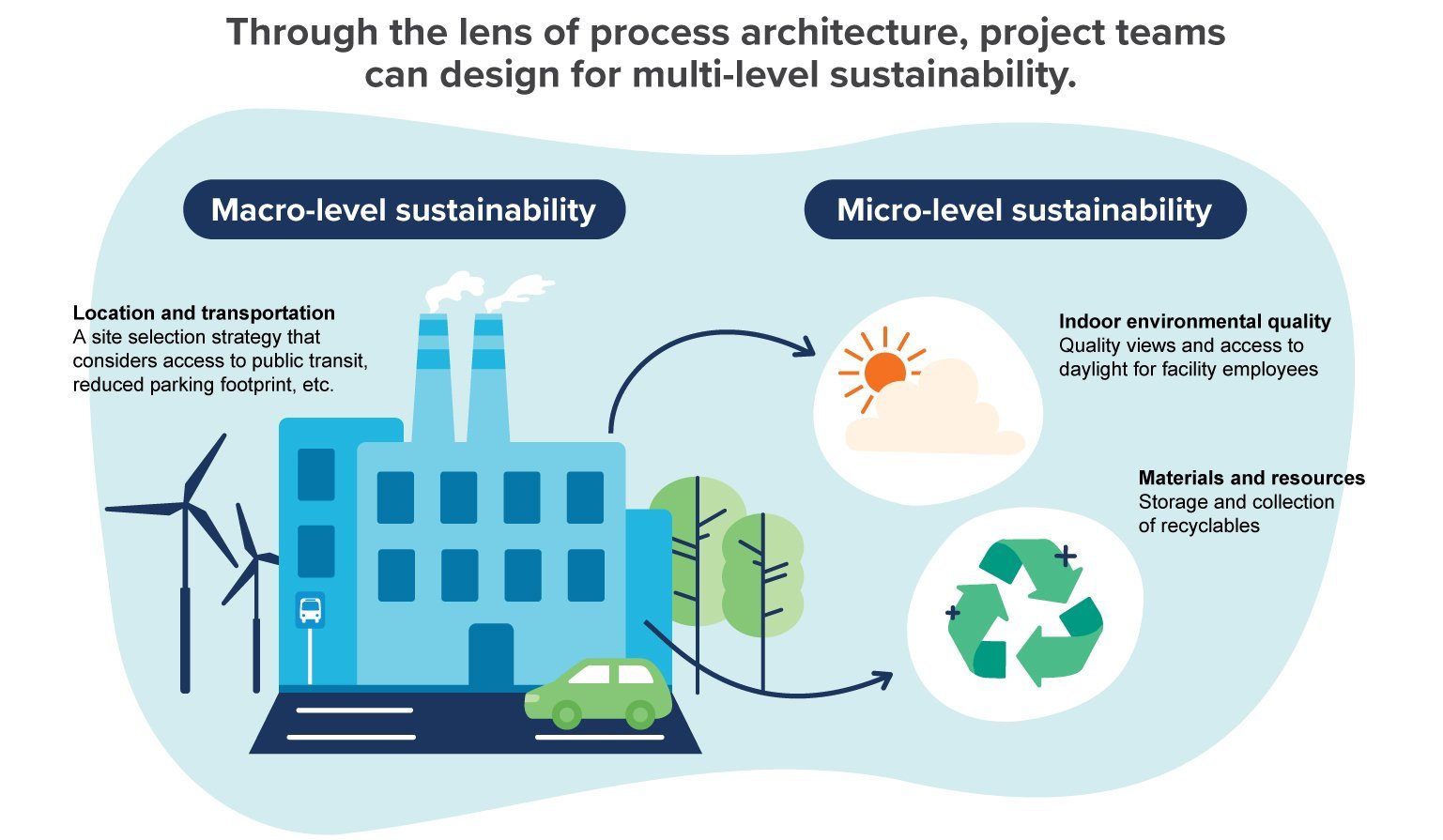
A more sustainable facility
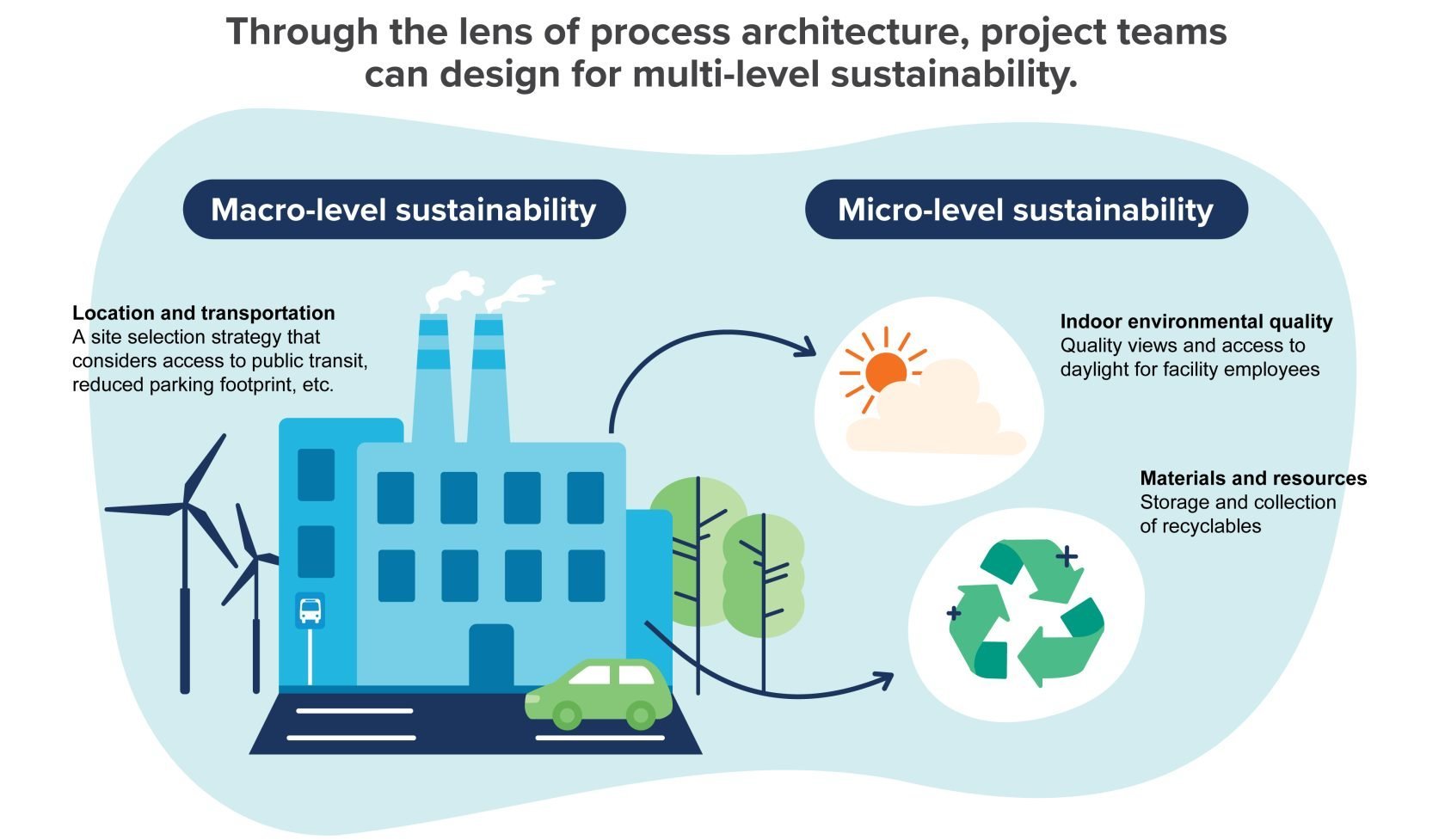
When implementing sustainable designs and strategies, many project teams focus on familiar concepts like energy or water conservation. These initiatives are important, but with the right expertise on board project teams could go much further, helping even the most energy-intensive facilities reduce their impact on the environment and increase their overall efficiency.
Process architecture is the missing piece. It encompasses both a high-level perspective on sustainable design and a ground-level approach, resulting in a building that maximizes sustainability from multiple angles, as advocated by the LEED rating system.
Read more: How to successfully plan and execute your next sustainable building design.