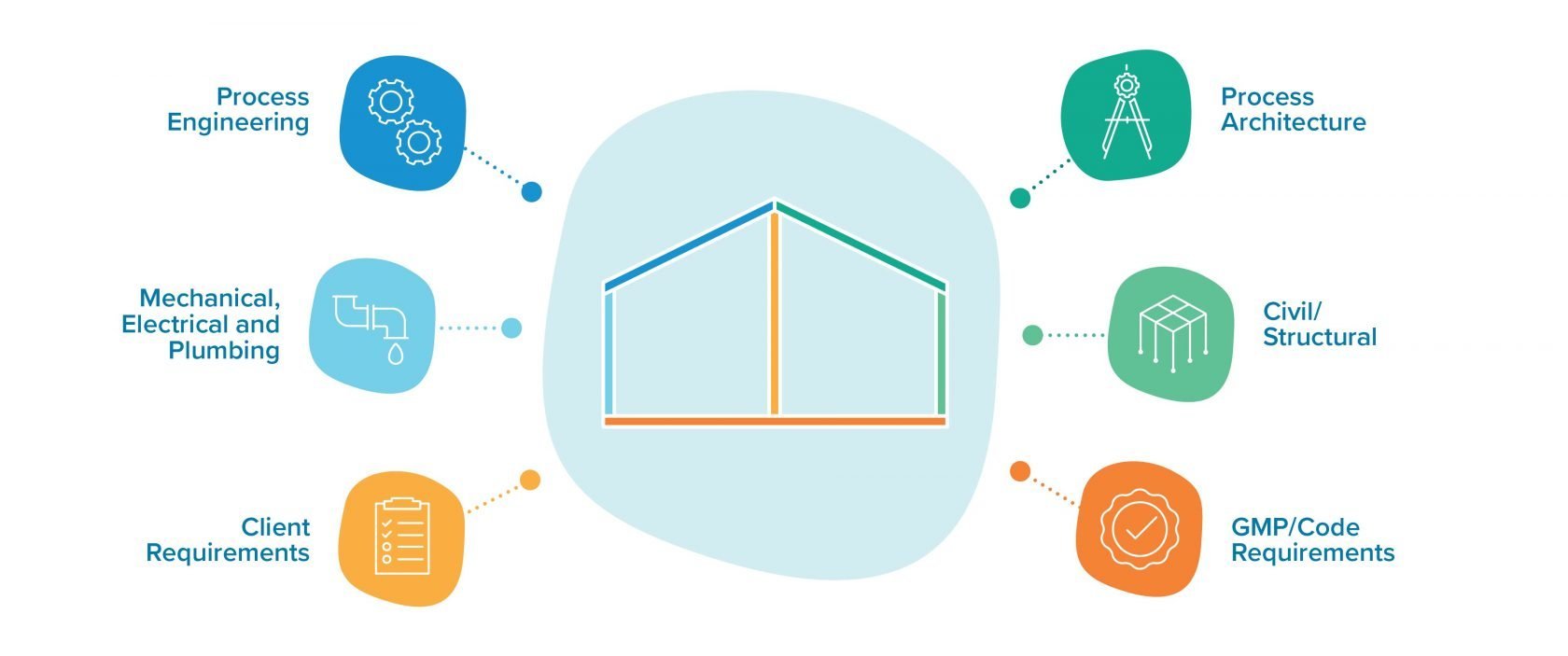
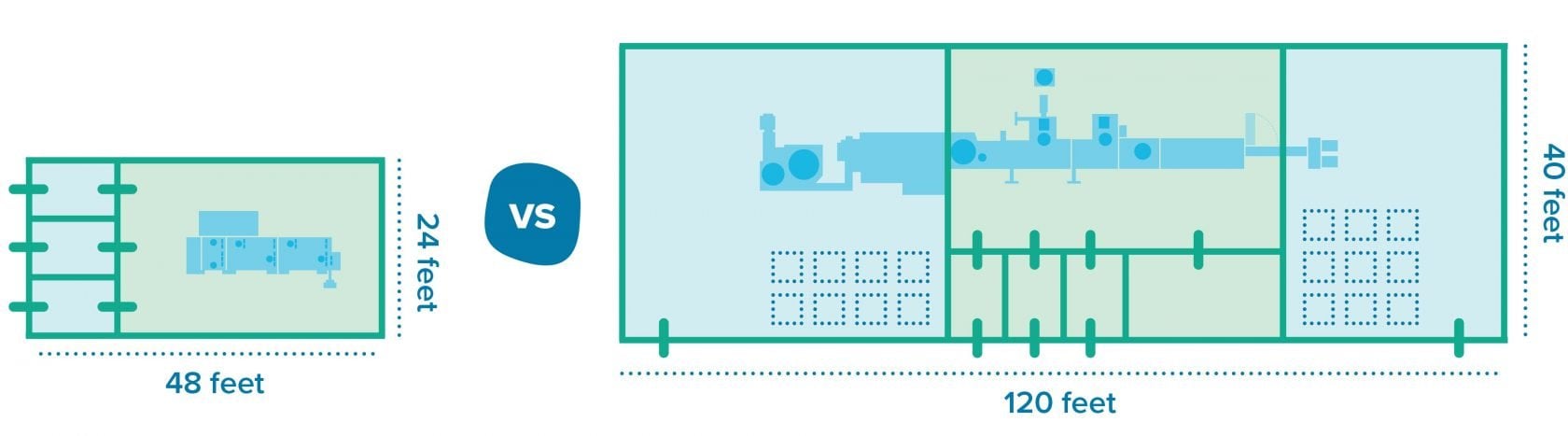
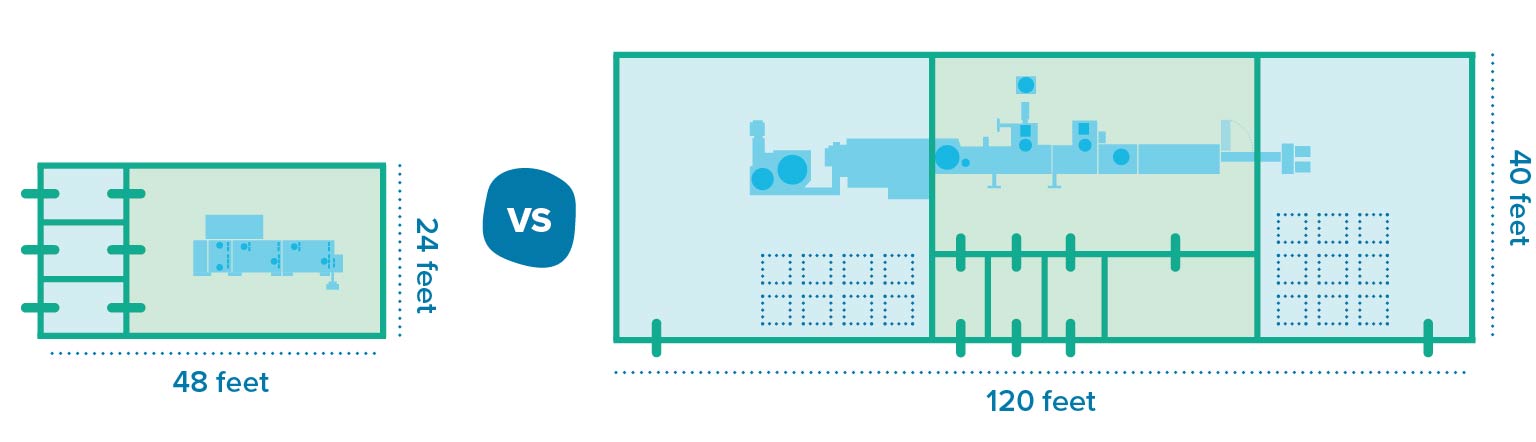
Instead, there are many levers to pull, each with its own escalating impact on how a facility operates. A process architect can work alongside process engineers, mechanical teams, and everyone involved in the delivery lifecycle to understand which of those levers to pull. From a business perspective, the goal is to:
Navigate impactful business decisions, such as ready-to-use vs. bulk components.
In contrast to the bulk alternative, RTU components require relatively little preparation and support areas, which leads to a smaller footprint for cleanrooms. These benefits come with tradeoffs, though. Storing RTU components requires more space compared to bulk components, for example. Then there’s the cost of goods: manufacturers spend more per vial when RTUs are involved, which could become unsustainable at large volumes.
Working with the project team, a process architect can help manufacturers weigh this business decision through the lenses of capital expenses, operational expenses, and cost of goods.
Understand the project’s capacity requirements.
What are the facility’s throughput goals during its first year of operation? What about year five, ten, or twenty? With insights from their collaborators on the project team, process architects can help business owners establish an appropriate internal or external expansion strategy based on their capacity requirements over time and translate that strategy into spaces allocated for future development.
Use that understanding to establish guardrails and guide project decisions.
Because they are deeply familiar with the project’s long-term commercial vision, process architects can help project teams design appropriate support and ancillary spaces. A parking lot that’s scaled for today’s workforce, for example, could become a serious constraint in five years, when the owner anticipates doubling the facility’s workforce.
The process architect can help project teams ensure that the facility can accommodate all of its required functions within its fixed footprint, now and far into the future. That could mean, for example, evaluating the manufacturer’s shift strategy to right-size its parking lot and cafeteria, or optimizing its warehouse by relying on just-in-time deliveries from an offsite location.
Deliver a facility that balances short-term needs with long-term flexibility.
During a facility’s lifetime, it’s likely that new products, technologies, and aseptic processes will emerge. How can today’s project teams help owners ready themselves to seize these new opportunities and generate value from their investment for decades to come?
The process architect can help to answer this question. They collaborate with project delivery experts to think through details such as ceiling height, hallway dimensions, level changes—anything that may constrain future changeovers or inhibit the speed and flexibility with which a facility can adapt, grow, and meet the needs of a rapidly evolving market.
How do process architects help to generate greater operational efficiency?
This is about what your sterile and aseptic manufacturing facility CAN BE.
Your process architect has the expertise to visualize design documents as living, breathing spaces through which people, materials, manufacturing equipment, and product flow. These visualizations, and the bottlenecks that they uncover, are one of the project team’s most powerful tools for building efficiency and flow into the final design.
A key component of the process architect’s approach is understanding the relationship between efficiency and compliance. Consider, for example, the costs and benefits of meeting stringent regulatory requirements through engineering-based solutions versus procedural design.
Engineering control: By “engineering out” the risk of contamination, owners can be assured of a safe and robust aseptic manufacturing operation—but they’ll find themselves operating a facility that’s much larger and more expensive than necessary.
Procedural control: It takes much less space to manage contamination via operating procedures. From a regulatory perspective, though, an overdependence on procedural control elevates the risk of contamination.
A process architect can find the ideal balance. The key to launching a facility that maximizes its risk management approach while minimizing inefficiency is to strategically leverage both control mechanisms. A process architect, with their view of the facility as a global system of interconnected parts, can help you refine this approach until it’s perfectly tailored to your unique regulatory and efficiency needs.
For example, consider using temporal segregation in bidirectional airlocks. This strategy can save space when footprint is limited, and it can reduce HVAC loads and minimize circulation spaces within processing areas. For temporal segregation to pay off, though, it needs to make sense in terms of risk to process and operational cadence.
That’s where a project team that includes process architects can provide valuable guidance. They can assess when temporal segregation makes sense, such as in an advanced therapy medicinal product (ATMP) filling operation with small throughput batches at a low cadence, and when it could be problematic, such as in a vaccine filling operation that must handle large, high-cadence batches.
How do process architects impact a facility’s look and feel?
This is about what your sterile and aseptic manufacturing facility WANTS TO BE.
In addition to balancing regulatory, commercial, and operational efficiency strategies during the design and delivery of an aseptic manufacturing facility, the project team is also thinking through how end users will experience that facility—and what impact that experience might have on critical factors like compliance, productivity, safety, labor retention, and corporate image. The process architect can help the project team assess these factors and their potential effects.
One of the best examples of this mindset in action is the concept of adding glass walls to aseptic production spaces. On its surface, this decision may seem like nothing more than a cosmetic flourish, but a process architect would integrate that glass wall in the right way to offer multiple benefits: