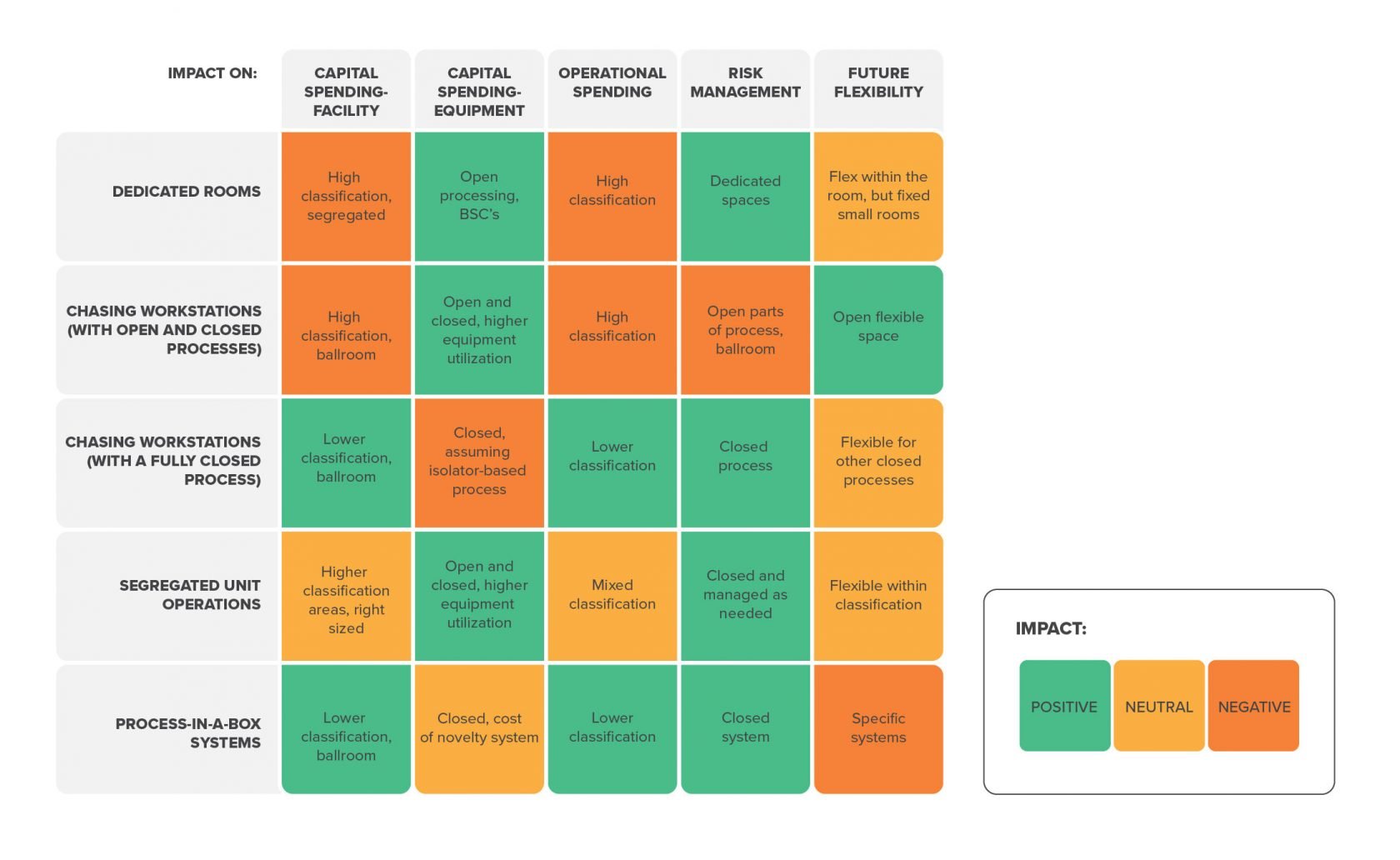
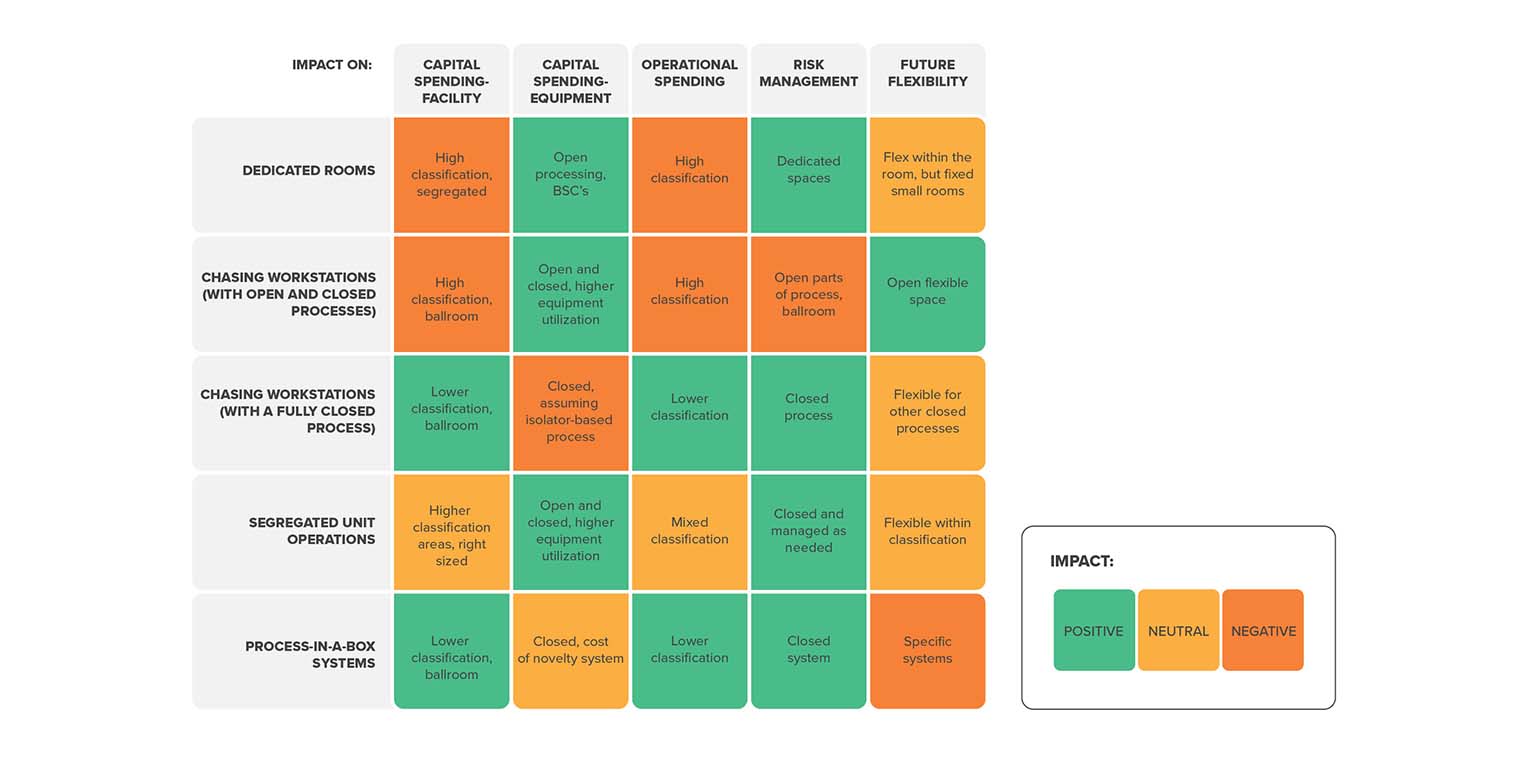
This approach combines elements of the first two layouts. Product batches move between higher- and lower-classified spaces as the process unfolds. Open process steps are contained to dedicated suites with a Grade B background, while operations that leverage closed processing equipment take place in a more cost effective, lower-classified ballroom-style space.
This approach may suit your operation if:
- Lowering your capital costs is an important business priority.
- You want to integrate both open and closed processes without overpaying for unnecessary or underutilized cleanroom environments.
- You need more flexibility than you’d be afforded if using an isolator (which requires you to identify open operations upfront in order to right-size the isolator).
Challenges to consider:
- What you save in upfront capital spending may cost you in terms of future flexibility. If you someday wish to close your entire process, for example, you may face a costly renovation to remove the walls, doors, and other elements of this segregated facility approach.
- Any degree of open processing will cost more per square foot than fully closed systems (although this approach reduces those costs by localizing highly classified environments to the spaces that absolutely need them).
4. Emerging: Process-in-a-box systems
An end-to-end, single-point, automated process-in-a-box platform will resolve most of the challenges explored above and radically change cell therapy manufacturing.
The idea is to take the concept of a product-dedicated suite and apply it to a fully closed piece of equipment. That all-in-one piece of equipment removes the risk of the cleanroom and opens the door to a leaner labor model, greater quality control, and virtually no risk of contamination.
From a facility perspective, this concept is game-changing. Rather than building, validating, and maintaining whole cleanrooms, it’s possible that manufacturers could contain their process-in-a-box system inside small-scale, fit-for-purpose environmental chambers. This would open the door to a whole different kind of manufacturing space—one that features densely stacked, fully closed systems inside a warehouse or a hospital wing, with no need for the traditional cleanroom.
This approach may suit your operation if:
- Quality control and risk management by process closure are your top priorities.
- You are investing in adaptable, next-generation capital projects designed to hold their value in the long-term.
- You are interested in reducing your facility and labor costs while rapidly scaling out your small-batch production pipeline.
Challenges to consider:
- Early adopters can find it difficult to align their unit operations with current process-in-a-box capabilities. This often results in a hybrid approach in which product batches move between automated steps inside the equipment and manual steps outside of it, or between pieces of closed equipment that are better suited for specific operations.
- A facility designed for process-in-a-box systems can’t be easily repurposed, and manufacturers may find it difficult to modify existing processes or to add new products that can’t leverage the closed and automated equipment platform.
- Vendors are continuously evolving their process-in-a-box strategies in response to these challenges. As new iterations emerge, cell therapy manufacturers will find opportunities to unlock the full potential of these closed and automated systems.
Which manufacturing approach is right for you?
No two manufacturing strategies are the same. Each unique approach requires significant time and expertise to get right. But from a high-level perspective, a few generalizations emerge that may help you begin to consider your own unique approach: