
Getting analytical on labs: analytical lab design trends
The past few years have yielded some interesting trends in analytical lab design and operation in the corporate biopharmaceutical marketplace. This article summarizes those trends and some considerations regarding the physical environment in which analytical labs operate.
1. Compartmentalization in analytical lab design
Research operations are increasingly compartmentalized. Lab waste, glassware, consumables, sample and material movement, instrument maintenance and calibration and other complementary functions are commonly handled by third parties on most major biopharmaceutical campuses. Analytical operations interfacing with these groups presents logistics, efficiency, security and bio-burden challenges. In some instances, third party costs will increase (e.g. manpower needs increase) if travel distances are too great or gowning and security logistics drastically reduce efficiency. Maintaining a clean environment requires clear segregation of travel for third parties coming from the outside, especially in biologically sensitive environments.
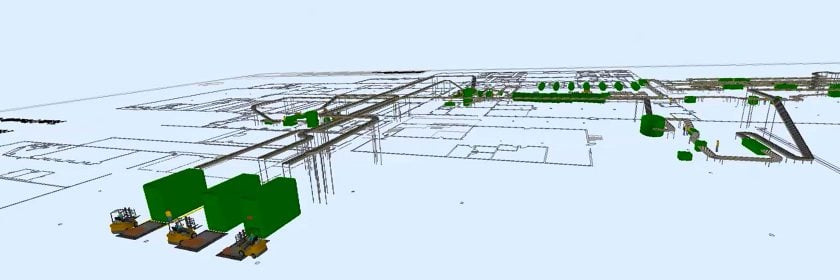
Figure 1 - Sample Lab Adjacency Diagram
The graphic to the right is a sample adjacency diagram from a recently completed consolidation project. As you can see, when mapping out future/renovated operations, you must consider much more than the lab itself, at times looking as far out as the loading dock and even into adjacent buildings.
The diagram also demonstrates the need to properly locate queuing points and other handoff or interaction zones between scientific personnel and various third parties. These nodes help address security and bio-burden concerns while simultaneously improving efficiencies for both sides.
Although the enclosed diagram represents a unique operation, the considerations are applicable across a spectrum of analytical groups and clients. Moreover, it stresses the importance of including leadership or representatives from the various third parties in your initial programming and planning sessions.
2. Process-centric vs. project-centric analytical lab design
Market pressure for return on investments in R&D has, in some instances, driven a shift from analytical operations being process-centric (e.g. where operations personnel concentrated on a step or sub-process of the overall analytical workstream) to being more project-centric. In the project-centric environment, manpower is streamlined (i.e. reduced) and cross-trained over various technologies and those same individuals are tasked with the end-to-end completion of the analysis, not just an individual step.
This project-centric environment where personnel stay with the sample from receipt/intake through testing completion drastically changes the flow within the lab. This needs to be addressed in analytical lab design. Although multiple queuing areas within the lab now go away, travel paths and shared instrument utilization become critical analysis points.
The figure below is a screenshot from an analysis performed on an analytical testing lab. The client was interested in modeling their current lab operations to determine equipment and personnel impact from a pending increase in sample volume.

Figure 2 – Testing Lab Optimization Graphic
The graphic represents a blend of our lab planning and operations improvement expertise and allowed for a data-driven analysis of testing equipment utilization, test-prep area congestion, travel paths and sample handling efficiency.
The analysis demonstrated that with some simple process flow improvements no additional staff, instruments or lab renovations were required to handle the increased sample volumes.
From this analysis, it was determined that:
- The lab could handle insourcing (163% additional testing throughput) with their current labor and equipment.
- With two additional fume hoods and an additional analyst, the new product could be accommodated (including the insourced tests, a total of 230% additional testing throughput).
Without this analysis, the client wouldn’t have been able to make a data-driven decision to keep outsourcing tests or buy new lab equipment, etc. In effect, there was a cost avoidance (by not buying new lab equipment that wasn’t needed) and a cost savings by insourcing tests.
3. Contract employees and standardized testing
Numerous biopharmaceuticals use in-house contract employees to execute standardized analytical testing procedures. These tests are still critical to research and development operations and their location still requires similar adjacencies to the balance of non-contracted testing; however, the integration of those testing functions carries a unique wrinkle. Although mostly an organizational structure issue, some interpretation of labor laws suggest these contracted personnel to be managed and operate somewhat independently of non-contracted (i.e. – full time, direct-hire employee run) operations.
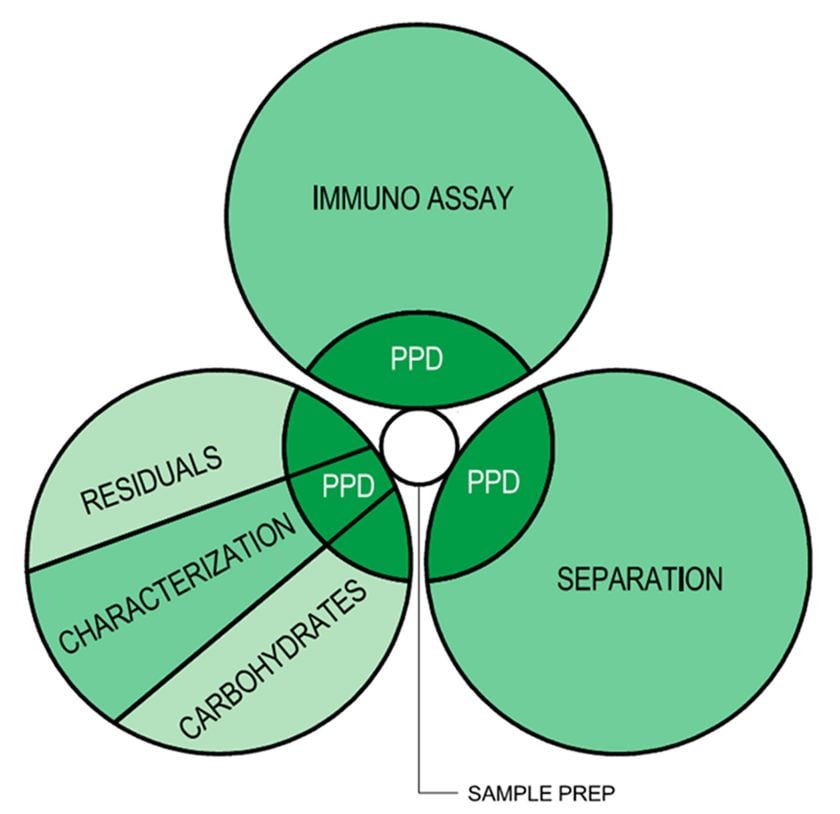
Figure 3 - Analytical (w/Contract) Adjacency Diagram
Whether contract or not, it is more efficient to cluster personnel based on technical objectives, especially in analytical environments where standardized tests are most commonly developed by full time, direct-hire employees. Otherwise you spend more capital on additional square footage for segregating these spaces and providing additional egress space.
The graphic at the right represents an analytical testing group who, in this instance contracted with PPD. As the graphic shows, there are more layers to the planning of their new facility than what would have been expected five years ago. A balance is struck between co-locating contract employees and clustering analytical functions. Again, much like with the third party coordination issues, its best to include representation from the contract organization in your early planning/programming effort.
4. Density, chemical quantity

Figure 4 – High-Density HPLC Lab
As testing becomes standardized and testing volumes increase, equipment quantities drive more and more dense laboratory environments. Although not as chemically heavy as some of their discovery/chemistry peers, analytical laboratories are finding themselves pushing the limits of allowable solvents. Traditionally, in a larger lab program, heavier chemical/solvent users were placed on lower floor levels where code-driven volume limits were less stringent. Analytical functions fell outside this “heavy user” classification and therefore were free to be placed on higher floors.
Based on more dense configurations, like the one in the image to the left, stored chemical quantities can quickly approach the limitations of those higher floors. As shown in the picture, each instrument uses dedicated solvents and dedicated waste collection. Amplifying the issue is the consideration by many biopharmaceutical EH&S departments that a waste container is “full” even if only partially full. The density of equipment and method of chemical calculation has the potential to eclipse the quantity limit for the analytical function, especially if on a higher-level floor.
Working closely with EH&S personnel to properly quantify the actual chemical quantities present is a good first step. Increasing the frequency of waste collection and limiting solvent storage at local queuing points are other strategies to stay below the limit. Additional steps can be taken by defining the in-use versus storage quantities and making proper use of chemical storage cabinets. Another option involves adding addition control areas via fire rated walls, but this adds additional cost to the project. Lastly, consideration should be given to integrating a solvent collection system with a termination point remote from the control zone of the analytical environment.
5. Analytical development feedback loop
Analytical labs within the biopharmaceutical market are always part of a bigger operation or business unit. Best practices developed within analytical testing operations are being sought after by peer organizations. The desire to have multiple peer groups in close proximity to analytical functions and the accompanying knowledge-sharing that results puts pressure on early planning phases to ensure proper adjacency and connectivity.
In a larger R&D planning effort, this became a challenge of scale. Several distinct development groups requested close adjacency to their analytical peers for the benefits listed above. Each development group’s lab footprint (qty. 6) ranged from 6,000 to 10,000 square feet. When blended with their office and support requirements, the net technical program exceeded 150,000 square feet. Clearly, to achieve the adjacency desired across the board, horizontal and vertical connectivity would need to be considered.
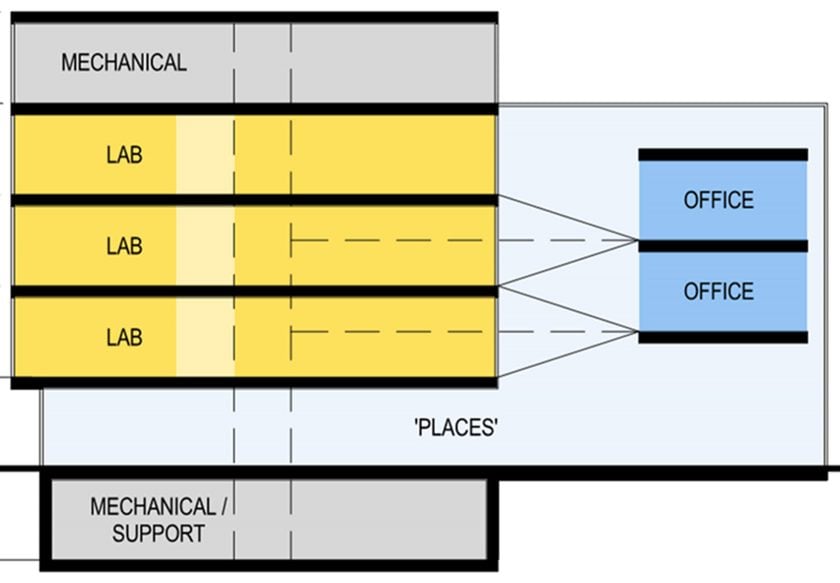
The graphic at the right is a conceptual representation of one of the proposed solutions taking into account a couple of program-specific factors. First, the ratio of lab to office was approximately two-to-one. Second, the scientific personnel split their time fairly evenly between the lab and office. With these considerations in mind, a split-level approach to lab and office adjacency affords both co-location of analytical staff with other groups in the office area and only a ½-level separation between analytical lab operations and both office areas. Located on the middle lab level, there is direct visual connectivity between all office functions and the analytical lab.
6. Planning for the unknown
Most analytical testing functions have limited insight beyond what compounds or materials they currently handle, but likely need to plan for the unknown. With the market calling for aggressive treatments and the corresponding compounds in the pipeline, highly potent materials are likely to cross the lab threshold and facilities need to be equipped (or quickly retrofitted) to safely handle them. The analytical lab design must accommodate these future requirements with pre-planned infrastructure and future spatial functionality.
The right balance between increased installed cost, pre-planned infrastructure and elongated downtime comes from detailed discussions during planning/programming. Conversations should cover classifications and quantities of materials, alternate forms of testing, and future instrument and equipment purchases. Equipping the lab on day one with anterooms, advanced filtration, segregated exhausts, washable surfaces, misting showers or other components is likely not the right answer. Ignoring potential future needs could result in significant renovations and downtime of the lab when then need arises. Installing a limited amount of infrastructure (HVAC, Electrical) and pre-planning some future floor space (anterooms, isolators) is a wise yet minimal investment to be pre-positioned for the future.