5 current trends that will shape the life sciences in 2021 and beyond
Many of the life sciences trends that are driving innovation today were here pre-COVID, but the pandemic was an accelerant. For all that it took, it gave life science researchers and developers new momentum, and from that momentum, hope.
This is true of the vaccine research that’s moving at blistering speed to curb this acute global crisis, but it doesn’t stop there. The same tide is lifting all areas of the life sciences industry. Projects that began as radical ideas in the research lab are now progressing through advanced clinical trials, propelled by novel life science technologies and processes, modern facility design, and new approaches to exchanging knowledge and attracting investment. This is particularly true for advanced therapy medicinal products (ATMPs); in 2020, for example, companies in the life sciences sector raised $19.9 billion, exceeding 2019’s investment by more than 200%. For patients who depend on this research to extend and improve their lives, there is new reason to feel optimistic about the future.
Here are five ways that this new reality will play out for the life sciences industry in 2021 and beyond, helping to streamline the entire process from research to approval and delivery of the therapies that patients so desperately need.
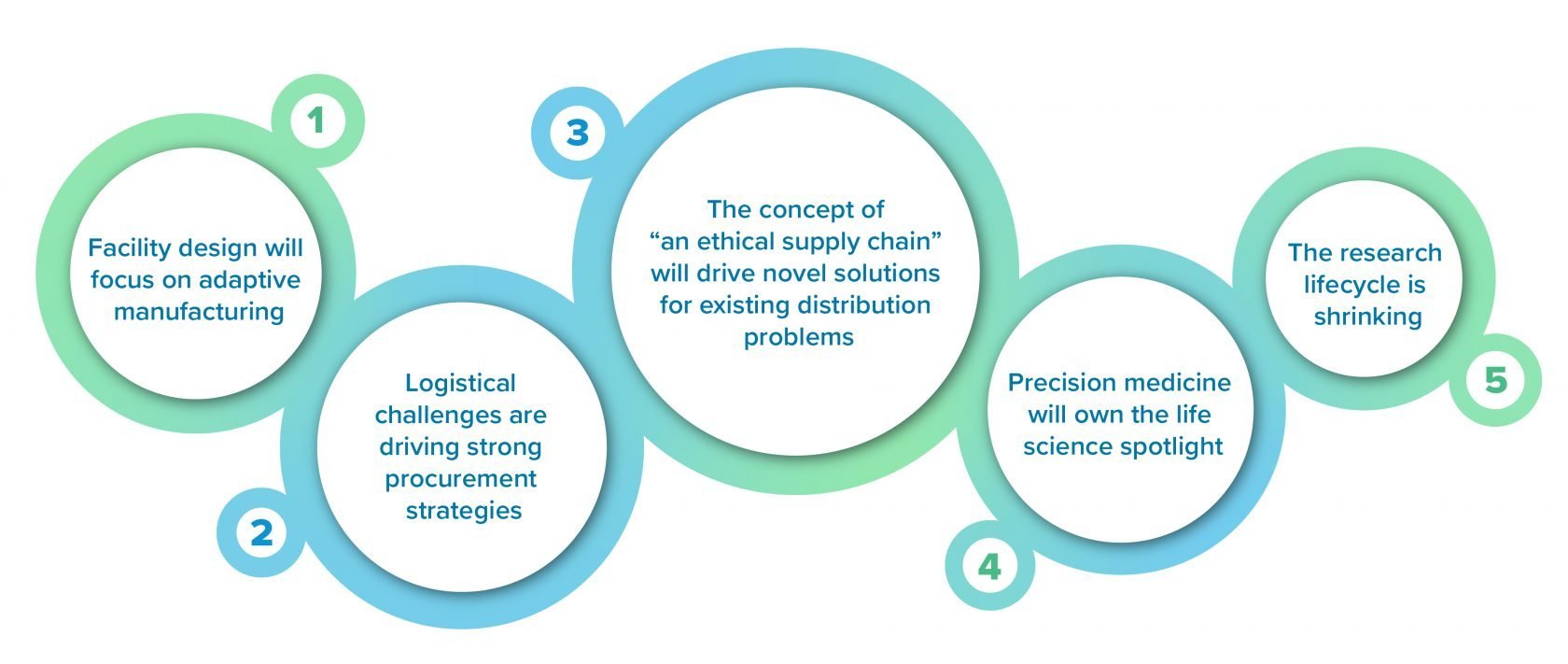

1. Facility design will focus on adaptive manufacturing
To move fast, you must be flexible. We saw this principle play out on a global scale when oncology researchers joined the fight against COVID-19, adapting their mRNA platforms to help develop an effective vaccine. This ability to pivot in response to any number of variants—a shift in demand, a breakthrough in life science research, a new technological approach—will become an increasingly important determinant of success as life science companies plan their way forward in an unknown and quickly changing world.
As a result of this emphasis on flexibility, the traditional, dedicated facility is giving way to life science facilities designed to manufacture multiple products or modalities. This means that where project owners once followed a straight line from the process flow diagram to the building’s layout, they must now design for the unknown, taking into consideration a holistic set of factors that include business objectives, research pipelines, sensitivities to risk, and emerging technologies. By the time they’re complete, these new facilities may well host processes and/or equipment platforms that haven’t yet been invented. To get the most from their capital expenditure, project owners need to ready their life science facilities for these changes by building agility into their layouts, process designs, and equipment platforms.
Emerging approaches to capital project delivery that emphasize agile and scalable manufacturing solutions are pushing more linear approaches aside. At CRB, we’ve answered this challenge with a suite of templated, product-agnostic cleanroom modules we call SlateXpace. As the life sciences industry grows more comfortable with the concept of adaptive manufacturing, solutions like SlateXpace will expand to provide life science innovators with more ways to move fast by staying agile.
2. Logistical challenges are driving strong procurement strategies
The problem of a constrained global supply chain for the life sciences isn’t new, but it worsened in the era of COVID-19. Social distancing and other pandemic-related interventions forced factories to reduce their production of stainless steel, glass, and other essential building materials—at the very moment when those materials were in peak demand. As a result, any drug developer seeking to build or renovate a licensable GMP facility today faces enormous competition for dwindling supplies. That goes for life science equipment and support materials, too, including single-use mixers and consumables, pre-sterilized vials, syringes, protective equipment, and much more.
These challenges have ignited important changes in how capital projects are scoped, scheduled, and budgeted. Leading AEC experts are modernizing their project execution strategies in order to address these chronic supply chain challenges in integrated and proactive ways. This means balancing the need for agility with the need to select, test, and preorder long-lead equipment and materials early in the design process, so that when construction finishes, project owners have a life science facility that’s ready for immediate validation and operation.
3. The concept of “an ethical supply chain” will drive novel solutions for existing distribution problems
The first approved COVID-19 vaccine from Pfizer and BioNTech, which requires ultra-cold (-70C) storage and shipping conditions, shines a spotlight on the importance of cold chain logistics. In North America and Western Europe, where a complex and reliable cold chain network has supported the distribution of biotherapeutics for decades, it’s easy to forget that this capacity simply doesn’t exist in large parts of the world. The division between rich countries and everyone else isn’t a new problem, but COVID-19 has forced us to pay closer attention to it, and with that attention comes the possibility of change for the life sciences industry.
The “ethical supply chain” is one that leverages new technological and societal solutions to promote equitable access to life-saving drugs. Entities like COVAX, a pillar of the WHO’s Access to COVID-19 Tools (ACT) Accelerator, are working to ensure the fair distribution of vaccines, and distribution will get simpler as the research matures and new temperature-stable vaccine candidates emerge.
We need novel and sustainable solutions that support the distribution of all necessary drugs. Microneedles are one example; by making it safer and easier for people to self-administer an intravenous drug, this technology could help to distribute much-needed vaccines in communities with limited medical resources. This is just one potential solution to a pressing challenge—a challenge that isn’t new, but is receiving a new level of attention in the era of COVID-19. Drug companies and the networks on which they rely are under more pressure than ever to ensure equal access and fair distribution. It’s time to take action and uncover innovative and cooperative solutions.
4. Precision medicine will own the life science spotlight
Amidst all the bad news of 2020 came an exciting headline: Jennifer Doudna and Emmanuelle Charpentier won the year’s Nobel Prize in Chemistry for their pioneering work on the CRISPR/Cas9 gene-editing technology. It’s the first time that a pair of women have won the award, and it acknowledges major advances in the science of curing previously incurable diseases and cancers. Precision medicine, already well-established, is about to hit its stride.
The speed at which this field is evolving is staggering, and it has broad implications for how life science innovators design, build, and operate a new generation of testing labs and commercial facilities. Rather than churning out repeatable formulations to meet the needs of a large population, these facilities will focus on the aseptic production of many very small, personalized batches—sometimes as small as a single dose. To make these facilities work both financially and in terms of GMP standards, project owners and their AEC partners must rethink their process design, equipment selection, cleaning, and decontamination protocols—everything.
Even the question of where to locate these facilities is complex; manufacturers need to consider their access to a transportation network capable of moving small, time-sensitive doses from the life science facility to the patient’s bedside. Add the logistics of finding a qualified workforce, managing waste, establishing adequate utilities, and many other site-specific details, and the challenge of identifying a suitable site becomes immense. All-new design solutions will play a key role in solving this challenge.
5. The research lifecycle is shrinking
The fight against COVID-19 prompted regulatory bodies, funding agencies, academic institutions, and private-sector drug developers to dismantle the walls that typically separate them. This cleared the way for researchers to sprint from sequencing the coronavirus genome in January 2020 to releasing commercially approved vaccines less than a year later. In 2019, this velocity would have sounded impossible; today, it’s poised to become the new normal, and it’s affecting every aspect of the life sciences industry.
Now that the world as a whole—and life sciences companies in particular—have had a glimpse of how quickly we can move when we all move together, there’s no going back.
To support this trend, the life science industry is embracing holistic and collaborative project delivery methods like design-build, Enterprise Project Management (EPM), and Integrated Project Delivery (IPD). At CRB, we responded to this trend early with a project delivery method we call ONEsolution. It’s based on the principle of radical team alignment; every last project stakeholder, from research scientists to trade partners to owners, belongs at the table.
The ONEsolution mentality of shared risk/shared reward helps us to control costs and improve speed, but it’s more than that. It’s about gathering the right people, giving them the right tools, and getting everything else out of the way so that the project can succeed. ONEsolution and other lean and collaborative project delivery methods share this in common. They’re about providing life science innovators with the project delivery solutions they need so that those innovators can in turn deliver the treatments that patients need.
Speed and flexibility are at the forefront of current life sciences trends. Innovations like adaptive facilities and ethical supply chains enable advances in targeted treatments like precision medicine, as well as help shorten the research life cycle. All of this is progressing with the health and well-being of the global population in mind. The more we can implement solutions to benefit people—quickly and effectively—the better.
At CRB, we think holistically about the future of the life sciences and our clients’ place in it. We’re committed to deeply understanding the latest regulations, monitoring and assessing new and emerging technologies, and proactively offering practical, on-the-ground solutions that incorporate both our clients’ business objectives and the realities of a complex and rapidly evolving world of the life sciences. This is how we will succeed, together.