
From antisense advances to the development of RNA interference (RNAi) therapies and discovery of the CRISPR-Cas9 system, the frontiers of genetics are being pushed with oligonucleotide research and development. In this deep-dive article, we’ll get a better understanding of current commercial unit operations, how to identify a facility’s needs amidst a changing landscape, and how to manage the inherent risks of upstream and downstream processes.
Oligonucleotide terms to know
Antagomirs also known as anti-miRs or blockmirs are a class of chemically engineered oligonucleotides that prevent other molecules from binding to a desired site on an mRNA molecule. Antagomirs are used to silence endogenous microRNA (miR)
Antisense oligonucleotide (ASOs) are small-sized single-stranded nucleic acids and offer some advantage over siRNAs in terms of targeting both nuclear and cytoplasmic located lncRNAs. Based on their sequence homology, ASOs bind to their target RNA sequence inside the cells and bring about gene silencing.
Aptamers (from the Latin aptus – fit, and Greek meros – part) are oligonucleotide or peptide molecules that bind to a specific target molecule. Aptamers are usually created by selecting them from a large random sequence pool, but natural aptamers also exist in riboswitches.
Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)
A particular region of DNA that consists of nucleotide repeats and spacers.
CRISPR-Cas9 is an enzyme that is capable of cutting strands of DNA.
A simple yet powerful tool for editing genomes allowing researchers to alter DNA sequences and modify gene function by using a variation of the Cas protein complex and a guide RNA as a target.
Downstream processing is the series of operations involved in the recovery and purification of products of interest.
Nucleoside phosphoramidites are highly reactive derivatives of nucleosides that are the basic starting material in the oligo manufacturing process.
The gold standard method for DNA synthesis that has been used in the industry for almost 35 years. Since its discovery, its simplicity and high efficiency have allowed large volumes of oligonucleotide sequences to be synthesized up to 200 base pairs in length. Currently, it is the only commercially viable chemistry able to provide the volume of DNA required by the synthetic biology market.
Solid-phase synthesis is the synthesis of chemical compounds whereby the reactant molecule is chemically bound to an insoluble material and reagents are added in the solution-phase. Excess reagents can be easily removed, and purification achieved, by washing.
Upstream processing involves all of the unit operations involved in producing the desired pharma product at a commercially acceptable concentration and quality.
How do oligonucleotides work?
Oligonucleotides are short strands of genetic (DNA or RNA) sequences. The word is derived from the Greek word olígoi (meaning few or small), and nucleotide, the building blocks of nucleic acids—such as those in DNA and RNA. Often referred to as simply “oligos”, these molecules are one of the most important tools in modern day molecular biology.
Natural DNA consists of nucleotides, which are repeating units that form a chemical chain, linked together by the action of enzymes. DNA synthesis is therefore one of the most important discoveries of the last century, allowing oligos to be manufactured synthetically with modified nucleotides in order to improve their therapeutic effects. Such oligos are designed to bind to specific DNA or RNA sequences via complementary base pairing—just like naturally occurring oligos. Although oligos prevent faulty genes from making faulty proteins, they don’t actually edit the genome.
What are oligonucleotide drugs?
Oligo drugs are designed to prevent or modulate the expression of almost any gene as part of highly targeted treatment. The first really powerful examples are now on the market, although the first approved oligos are nearing 30 years old.
In recent years, considerable advances have been made in the design of oligo drugs, allowing for the development of therapeutics that have greatly improved stability. There has been considerable progress in overcoming the challenges related to administration, biodistribution, cellular uptake and undesired side effects.
Many of the modifications also provide greater specificity, leading to better efficacy and safety. The main challenge of oligonucleotides is efficient drug delivery. It is hard to get them anywhere but the liver, so the goal is selectively targeting the oligo to desired tissues. Various kinds of oligos required different strategies to ensure efficient uptake at the cellular level and release within the cytosol. As these challenges are overcome, many new forms of oligo drugs will reach the market. To date, only sterile injectable products can be formulated—although that too will change as research presses forward.
What are oligonucleotides used for?
Unlike the bulk of biologic drugs which target proteins (and thus the downstream effects of diseases), oligonucleotides target errors in the genetic code—the root causes of diseases. Oligonucleotides are used for rare disorders that have been previously untreatable, such as neural and neuromuscular conditions.
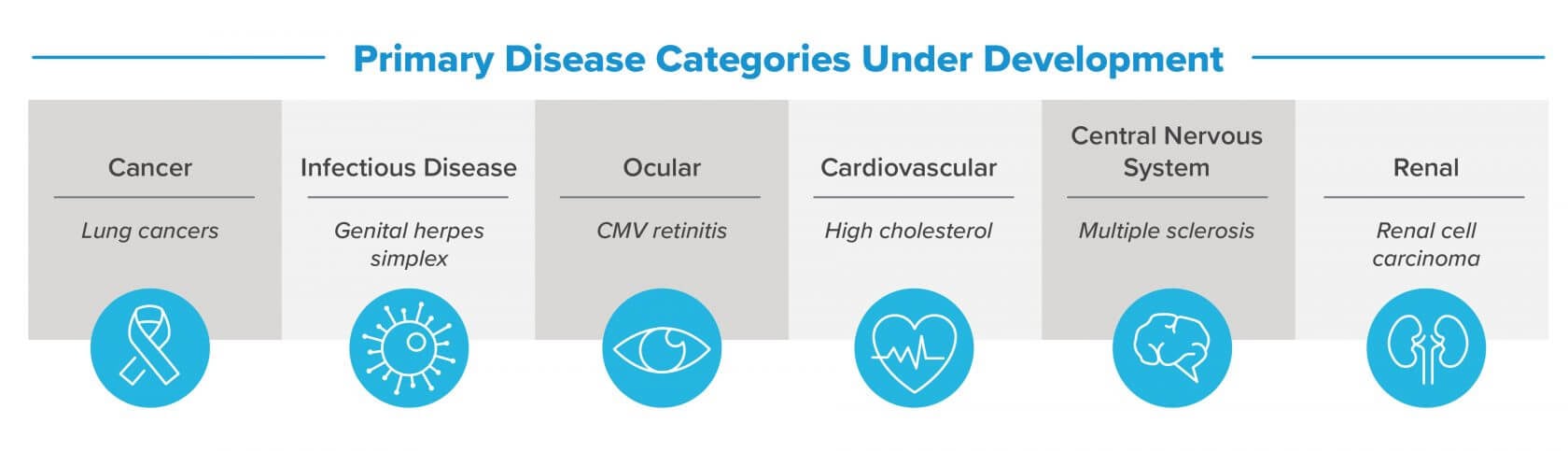
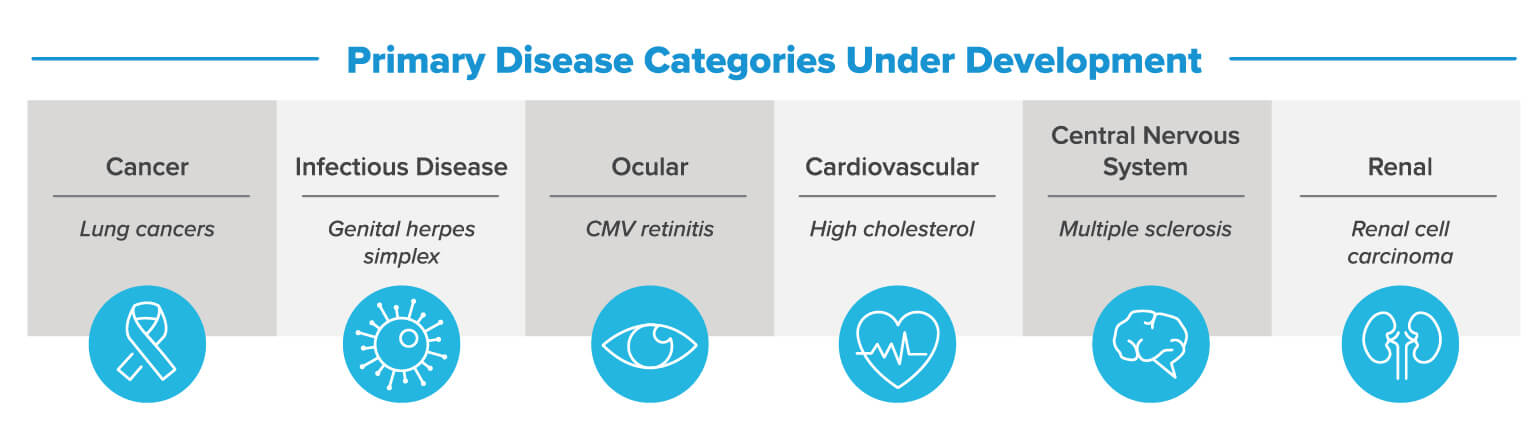
Several oligonucleotide drugs have received regulatory approval. Many more drug products are currently in late-stage clinical trials and moving toward commercialization. These exciting developments create the next challenge: the design and construction of oligonucleotide manufacturing facilities at a commercial scale.
Primary oligonucleotide manufacturing unit operations
The oligonucleotide field is still in its commercial infancy so it does not have decades of commercial-scale manufacturing experience on which to draw. Up until now, most oligonucleotide development has taken place in labs. As the field rapidly grows, manufacturers are transitioning from labs to large-scale processes. This necessary leap, however, brings significant challenge to unit operations.
The field of oligonucleotides exists at the intersection of chemistry and biology. In order to manufacture injectable oligo drugs at the highest quality possible, a facility design must therefore incorporate aspects of both small-molecule and biologic drug production to address the very different needs of the upstream and downstream unit operations.
All oligonucleotide manufacturing processes require the use of flammable, hazardous materials during upstream processing and then very clean biopharma processes during downstream processing. A facility must therefore handle large quantities of flammable liquid while also providing a suitable environment for sterile formulation and fill-finish of the final parenteral oligo drug product. Optimizing these facilities requires engineers and architects who can speak both of these languages and the corresponding regulations.
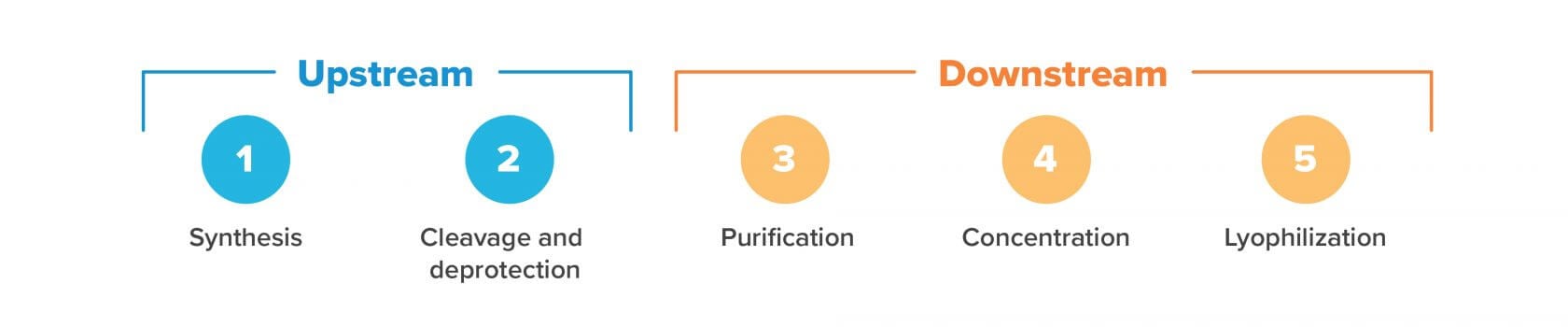
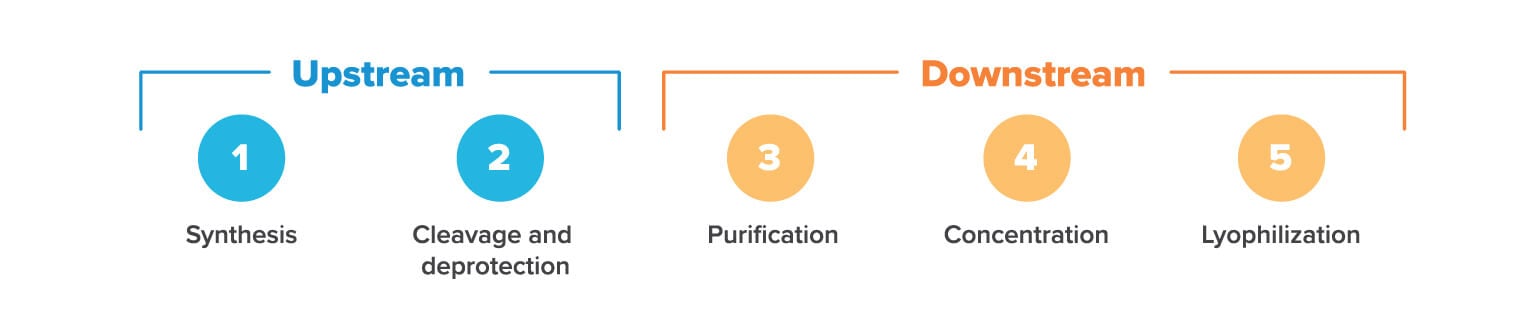
Upstream processing
The first few steps of oligonucleotide production use solvent-heavy processes that do not require clean rooms. At this stage, microbial contamination is not a concern as no living organisms (that we know of) can survive the harsh chemical environment. It’s only later on, in downstream processing, that there are concerns about bacterial contamination. Upstream processing includes synthesis and cleavage/deprotection, as we’ll discuss below.
1. Solid phase synthesis
The oligonucleotide drug process begins with a chemical reaction, which can be explained through phosphoramidite chemistry. The chemical reaction during synthesis is well understood and predictable. Scientists can model how to manufacture a specific sequence, while also accurately predicting where impurities will come from. (This specific kind of reaction is not unique in the pharma world, as it is also used in peptide production.)
Chemical modifications to the molecules:
- Enable high-efficiency binding during synthesis
- Enhance their ability to bind to target RNA/DNA sequences
- Increase their stability and resistance to nucleases (important for downstream)
- Increase the probability of cellular uptake (more stable in the body)
The chemical reaction, or synthesis, of oligonucleotide reactions uses several thousand liters of flammable solvent per kilogram of drug substance produced. Common solvents include ethanol, toluene, and acetonitrile. Through a cycle of chemical reactions, individual nucleotide residues are coupled, creating the desired sequence. Through a repeated sequence of reactions, the chain of modified nucleotides grow rapidly.
Better reactions during synthesis result in better yields, but this is more difficult at a large scale than in a lab setting. Fortunately, there has been huge progress made over the last five to ten years through the improvement of raw materials. The solid-phase synthesis step, however, always comes with some yield loss.
2. Cleavage & deprotection
Once the synthesis sequence is complete, oligos are cleaved from solid support. After cleavage, the solution of oligonucleotide is heated in concentrated aqueous ammonium hydroxide to eliminate protecting groups from the bases and phosphates. Deprotection then removes blocks that are used to ensure linear chains versus split chains.
The cleavage and deprotection steps are performed using a variety of harsh chemicals, including concentrated ammonia, so operators must be protected from exposure.
Downstream processing
During downstream processing, there is migration from solvent to water-based solutions. Measures must be taken to minimize microbial contamination. This requires sterile formulation and fill-finish operations like those used for other types of sterile parenteral products. Many manufacturers tend to be conservative, electing to use isolators to ensure the highest level of quality and patient safety.
3. Purification
Downstream process flows vary significantly amongst various drug manufacturers—particularly in terms of the purification and final formulation requirements. The bulk of purification processes focus on the reduction of impurities significantly shorter or longer than the parent molecule.
Ultrafiltration/diafiltration (UF/DF)
The goal of UF/DF is switching the solution from one type of solution into another. For example, it may be in a solvent and needs switched into water, or it is in a buffer and needs to be switched into a solvent. UF/DF can happen before or after the chromatography step, depending on what kind of oligo is being made. The oligonucleotide is often maintained in an alcohol solution or a mixture of organic solvent and water until the final stages of the purification process to reduce the risk of microbial contamination.
Chromatography
Chromatography weeds out large outliers in chain length and is part of the purifying and isolating process. Various methods (IEX, RP-HPLC) and modes (step, linear) may be used, depending on the manufacturer’s goals during this step.
4. Concentration
Regardless of the purification technique used in the previous step, oligonucleotides must be isolated from the purification buffer and, sometimes, converted into an appropriate salt form. This final isolation step is done for stability, logistics, and operational convenience.
Concentration may use ethanol precipitation or UF/DF as intermediate isolation options. In comparison to ultrafiltration, which can accommodate nearly any purification buffer system, ethanol precipitation has more limitations. Therefore, the method of concentration will be inextricably linked to the method and mode of chromatography used in the previous one.
5. Lyophilization
Although most oligos are stable at room temperature, the most common current practice is to lyophilize (freeze-dry) the drug substance before formulation.
Oligo facility setup
Once a production facility is properly configured, it can be used to manufacture many different oligo sequences. The platform technology is easily scalable and the same piece of equipment can be used to synthesize any number of oligo drugs. While single-use technologies are not yet widely used for formulation and fill-finish processes, they will likely be adopted as the oligo drug field matures.
Identify your needs
There is not yet an oligonucleotide drug requiring significant sufficient metric tons of products to warrant a compound-dedicated plant. Therefore, it’s important to build with flexibility in mind, as any manufacturing facility is most likely to be a multi-product one. User requirements are always a good basis of design, so start by asking:
- Do I need a facility that can do both oligo and biopharma? (requires clean boundaries)
- What types of oligos will I be making? (single-stranded, double-stranded, etc.)
Site selection
It can be particularly challenging to incorporate the necessary level of containment in facilities that are constructed within existing buildings. A standalone building is ideal. If a toxic spill occurs, it needs to be instantly vented to the outside of the building. In some regions, local authorities require that everyone within an ¼ mile must be evacuated when there is a spill. Therefore, multi-tenant facilities (such as strip malls) are not an ideal location for a commercial scale oligonucleotide manufacturing site.
Material selection
Most upstream and downstream equipment should be stainless steel. In equipment containing solutions with high chloride content where corrosion is a potential issue, higher alloys are used. Tubing, gaskets, and other soft equipment components must be made of elastomers that are compatible with a range of chemical solvents. Formulation and fill-finish equipment usually includes CIP/steam-in-place capabilities to minimize the risk of microbial contamination.
Storage considerations
Although oligo drugs are quite stable in neutral aqueous solutions, most manufacturers are taking a conservative approach and storing their finished products at colder temperatures. Perhaps, as more oligo drugs are approved and regulatory agencies become more familiar with their stability properties, the need for cold storage will be eliminated. For now, most facilities are taking a “better safe than sorry” approach.
Reduce waste
Currently, quantity and quality of raw material are of paramount importance in oligo manufacturing, but as demand increases, waste and solvent consumption are also worth consideration. As technology progresses, solvent recycling will likely eventually be incorporated to reduce waste and raw material costs.
Managing the risks of oligonucleotide manufacturing
Essentially, a commercial-scale oligo manufacturer is a chemical plant making a drug product—and its owners need to be prepared for the corresponding hazards and regulatory scrutiny. The largest risks associated with oligonucleotide manufacturing are due to:
- Expensive raw materials
- Flammable, hazardous solvents
- Risk of microbial contamination
Reaction dynamics
Notably, most of the deviations and process failures to date have been associated with equipment failure as opposed to a failure of the chemistry or process parameters. Simplification (by limiting the number of manufacturing steps) is one way to minimize process failure by reducing the number of potential mechanical failures and equipment errors.
Reaction parameters need to be controlled which requires temperature regulation and consistency. The solvent supply to synthesizer cannot have air bubbles, requiring a consistent pressure and flow rate. There also cannot be a temperature spike. Therefore, total control and steadiness of all of those reaction dynamics and characteristics is essential for safety during synthesis.
Facility planning
Risks can also be mitigated or eliminated through software, controls, and hardware. For example, minimize the risk of fire caused by inert gases by using fire-safe valves, air interlocks, blow-off panels, and unidirectional airflows. Areas with flammable solvents require electrical classification. Containment solutions for water released in the event of a fire must be included—which can have a significant impact on the design of a facility.
Activities supporting operation of the synthesizer require careful planning of the facility layout. In current facilities, the dozens of raw materials and large volumes of solvent must be funneled to that single piece of equipment. The spent solvent must then be removed as hazardous waste.
Operator safety
During synthesis, there is no risk of the operators contaminating the drug product but there could be great risk to the operators due to the explosive chemical substances in use. Therefore, it is essential for oligonucleotide manufacturers to meet all code requirements in order to minimize risk of any safety to operators. These include spill containment, electrical classification, and HVAC system code requirements.
Supply chain
Oligonucleotide manufacturing requires a well-dialed upstream supply chain. The major raw materials required for oligo manufacturing are hugely expensive. The solvents are also hazardous, which further increases the cost of transportation. The geography of the manufacturing site affects cost, with some remote clients struggling to source solvents due to exorbitant shipping costs. If the facility’s remote location poses a challenge to the supply chain, additional refrigerated warehousing space will be needed.
Drug purity
All solvents must be pure, raw materials. The presence of critical impurities in chemical preparations of phosphoramidites is an important regulatory and safety consideration during the oligonucleotide manufacturing process. Therefore, this aspect of the oligonucleotide manufacturing process requires a well-documented risk assessment.
Regulatory considerations
Only a handful of oligonucleotide therapies have been approved, so industry standards have yet to be established. Oligonucleotides’ size falls between that of small- and large-molecule drug substances, which raises questions about their classification, even for regulators. Oligos have been lumped in with APIs, but that is probably not a sufficient regulation of oligos. Common sense dictates that marrying a flammable and toxic compound handling facility with a facility that must be operated to hygienic standards requires unique solutions.
The biggest regulatory hurdle is answering this question: How do you know that you have exactly the right sequence? It can be difficult to get only the length you need as chromatography is based on molecular weight. Batches are done on the order of a mole, with billions of chains produced. It would be impossible to test every single molecule! This opens questions about risks to patients. For example, is a N+3 or N-3 simply not effective—or is it not safe? More clarification is expected as greater numbers of oligo drugs graduate from clinical trials into the market.
Onward into the future of oligos
The oligo industry, as exciting as it is, is still in its commercial infancy. The best is yet to come! Most of the companies involved in the manufacturing processes are banking on the success of the oligonucleotide platform to warrant further capital investment. Once scientists discover how to get these strands where they need to go in sufficient quantities, the floodgates of demand will open for these drugs. Let us help you prepare for the future of oligonucleotides!

