With cell and gene therapies in your research pipeline, you have the potential to impact patient lives in all-new ways. Before that can happen, though, you need a commercial facility that’s purpose-fit for the unique challenges of cell and gene therapy manufacturing like scalability, aseptic processing, and even staffing.
This may seem like obvious advice, but companies under pressure to move fast may not realize that they’re entering the site selection process with incomplete information. As a result, too many cell and gene therapy clients come to us with greenfield sites or core and shell buildings that appeared suitable at first, but reveal hidden constraints and costly surprises as we begin the design process.
The good news: you can avoid this mistake for your own cell and gene therapy capital project. All you need is the right expertise and, most of all, a commitment to upfront due diligence. Because that’s the thing about site selection—some of the most important decisions you make can happen even before you have a particular site in mind.
Before considering any sites
Step 1: Conduct a site-agnostic baseline study.
In this step, you’ll define your business and manufacturing baseline needs to identify your facility requirements, including overall square footage and site must-haves.
To navigate the complexity of scaling from a research lab to a CGMP environment, you need to establish a few crucial navigation points. That’s what this step is all about: understanding where you’re going so that you can get there along a straighter, more cost-effective path.
Start by defining your facility’s projected baseline throughput, both short- and long-term. Your front-end programming and design team can then use that baseline to scope your project at a high level. Deliverables during this step may include process block flow diagrams (BFDs), preliminary layouts, utility assessments, and more. At this point, you should also have a firm idea of your intended manufacturing approach.
This exercise will give you an early picture of what you need from your facility—and a chance to compare that picture with your business plan, making any necessary adjustments to ensure that your scope and budget are closely aligned.
Greenfield or renovation? Use your baseline study to decide.
In this article, we’re focused mainly on cell and gene therapy manufacturers seeking a core and shell building that they can program to fit their needs. However, a greenfield project may be a more appropriate pathway for some companies, depending on their long-term business needs.
Understanding the advantages of each option and how those advantages align with your baseline requirements and commercial vision is key. For example:
- A core and shell building may accelerate your speed-to-market, but a greenfield project will give you more design control.
- A greenfield project may require more upfront investment, but with the right planning, it could help to maximize your long-term returns.
- A greenfield project would maximize flexibility in terms of determining the facility’s horizontal and vertical configurations, whereas the existing core shell’s predetermined physical boundaries may be a constraint.
Your site-agnostic baseline study can help you explore these complex factors and set your project up for long-term success.
Step 2: Use your baseline study to establish a site selection checklist.
In this step, you’ll use the results from step one to define what you need from your site, what you can live without, and the degree to which you’re willing to compromise.
Now that you’ve programmed your facility on paper, you’re closer to finding it—or something close to it—in the real world.
To make that search easier, use your site-agnostic baseline study to draw up a checklist of specific criteria. The more detailed your checklist, the more useful it will be. Think in terms of ranges—what’s your minimum acceptable value for a particular metric, such as square footage, and what’s your maximum?
A typical checklist should include details such as:
Facility requirements
- Square footage: What is the minimum size you’ll need in order to meet your manufacturing targets? How far beyond that minimum are you willing to go (and to pay for) to maximize future expandability?
- Floor-to-beam height: How much mechanical stack-up space will you need above your cleanrooms? It’s not unusual for first-time CGMP manufacturers to underestimate this value, causing themselves significant headaches (more on that later).
- Warehouse logistics: How many loading docks will you need? To what degree are you planning to shore up your supply chain?
- Vertical arrangements: Are you open to a multi-level building with elevators?
Utility requirements
- Electrical capacity: Can the site handle the building load, including air handlers and emergency power?
- Mechanical infrastructure: Is the building structurally secure enough to support the necessary mechanics of a commercial CGMP operation?
Logistics requirements
- Transportation logistics: How close will you need to be to the patients relying on your therapies, or to a major transportation hub?
These criteria will help you eliminate facilities that aren’t suitable for your project and zero in on ones that have potential. If necessary, you can also rely on this list to help you determine which adjustments, trade-offs, and sacrifices you’re willing to accept to move forward with a particular site.
During the Site Selection Process
Step 3: Assess each site according to your selection checklist.
In this step, you’ll determine which site is most suitable for your manufacturing and business goals—and you’ll have clarity about the sacrifices and trade-offs that may be necessary to make that site work.
Some manufacturers jump straight to this step, but you’ve taken the time to establish your facility’s baseline requirements. You now have a checklist to help you quickly eliminate inappropriate options and conduct a transparent and realistic assessment of the remaining candidates. That puts you in an advantageous position.
Even with a well-considered checklist, though, it’s possible to have certain blindspots—especially if this is your first time transitioning to a CGMP facility, like many cell and gene therapy startups. These are some of the common blindspots we’ve noticed, particularly for those seeking to retrofit a core and shell building.
Overall Building Form
A rectangular layout is ideal in terms of supporting a simple, linear CGMP flow through your facility (think personnel, products, waste materials, etc.). Rounded walls may make for interesting amenity spaces, but you’ll have to square them off to create suitable cleanrooms, which means wasting valuable square footage.
The shape and overall design of a particular building could impact your construction strategy, as well. If you’re planning to install a wall panel system, for example, you need a shell building with adequate structural support—or else you’ll have to build a second structure yourself, which amounts to a secondary building inside the shell. Likewise, if your construction strategy relies on offsite fabrication, you’ll need a shell with the right dimensions and layout to accommodate pre-assembled modules.
Vertical Clearance Requirements
This is one of the most common miscalculations we see from cell and gene therapy manufacturers as they navigate the lab-to-facility transition. It’s easy to understand why: When it comes to small-scale benchtop processes like those common to autologous cell therapy manufacturing, there might not be much change between the research lab and the commercial facility in terms of manufacturing workstation space. So why should the ceiling height change?
The problem is an overfocus on what goes on below the cleanroom ceiling, without enough consideration for what goes on above it.
Unlike a research lab, a CGMP cleanroom needs to meet strict classifications in terms of air quality. For example, aseptic preparation and filling typically requires a Grade B cleanroom environment; to meet that requirement, you’ll need a mechanical system capable of filtering airborne particulates, controlling humidity and temperature ranges, and providing a continuous supply of clean air into the room. That mechanical infrastructure is typically stacked above the cleanroom ceiling, which takes up considerable space.
Then there’s the maintenance consideration. In a research lab, it’s not unusual for maintenance teams to access overhead mechanical systems directly through the ceiling. In a CGMP environment, that’s not practical. Access to the mechanical stack-up space should be segregated from the cleanroom environment to prevent contamination and reduce the need for rigorous cleaning. That means designing at least 6–8 feet of clearance inside the mechanical space in order to accommodate workers, according to the building code. Once you factor in the overhead ductwork, piping, and structural beams up there, that creates an even greater spatial requirement.
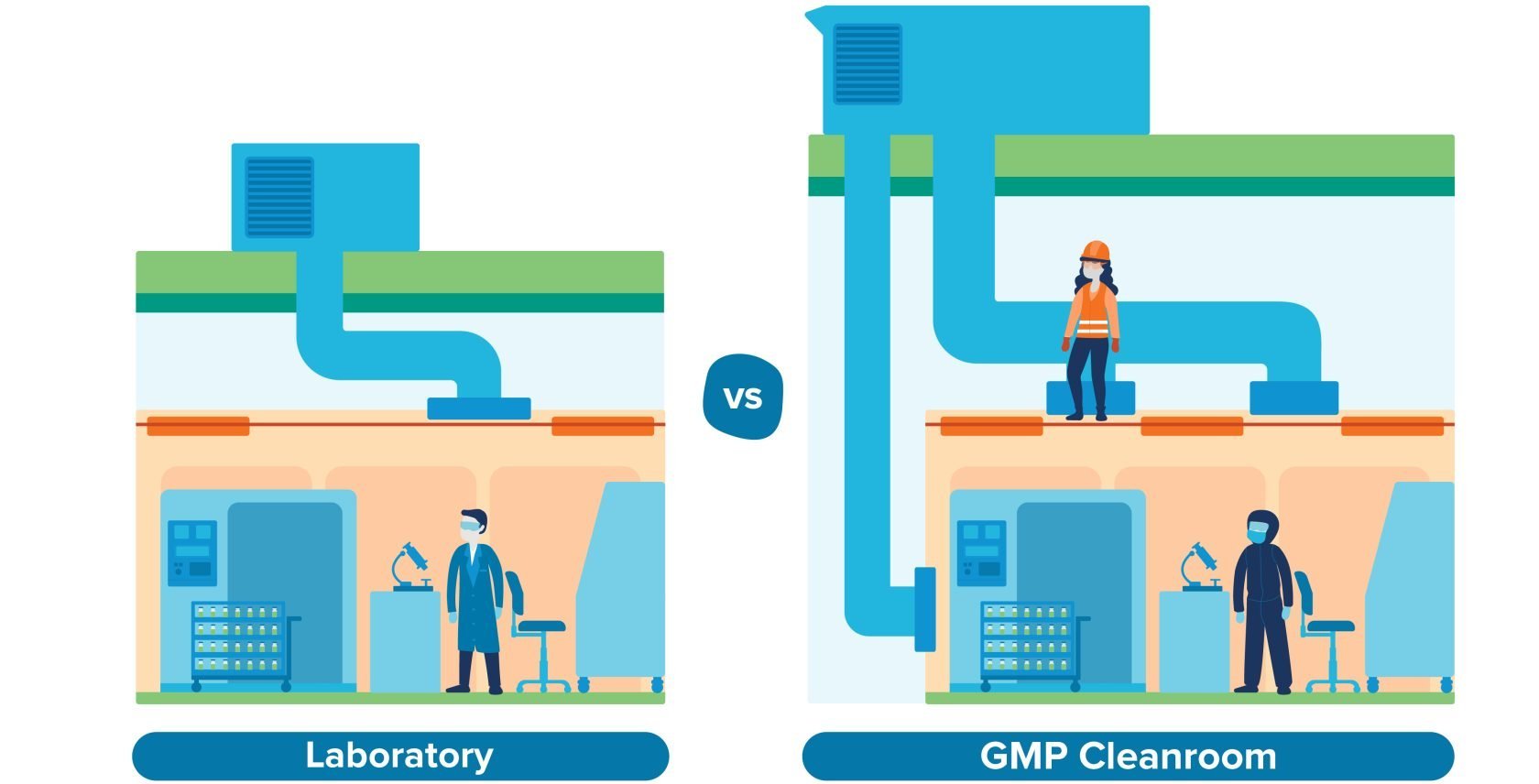
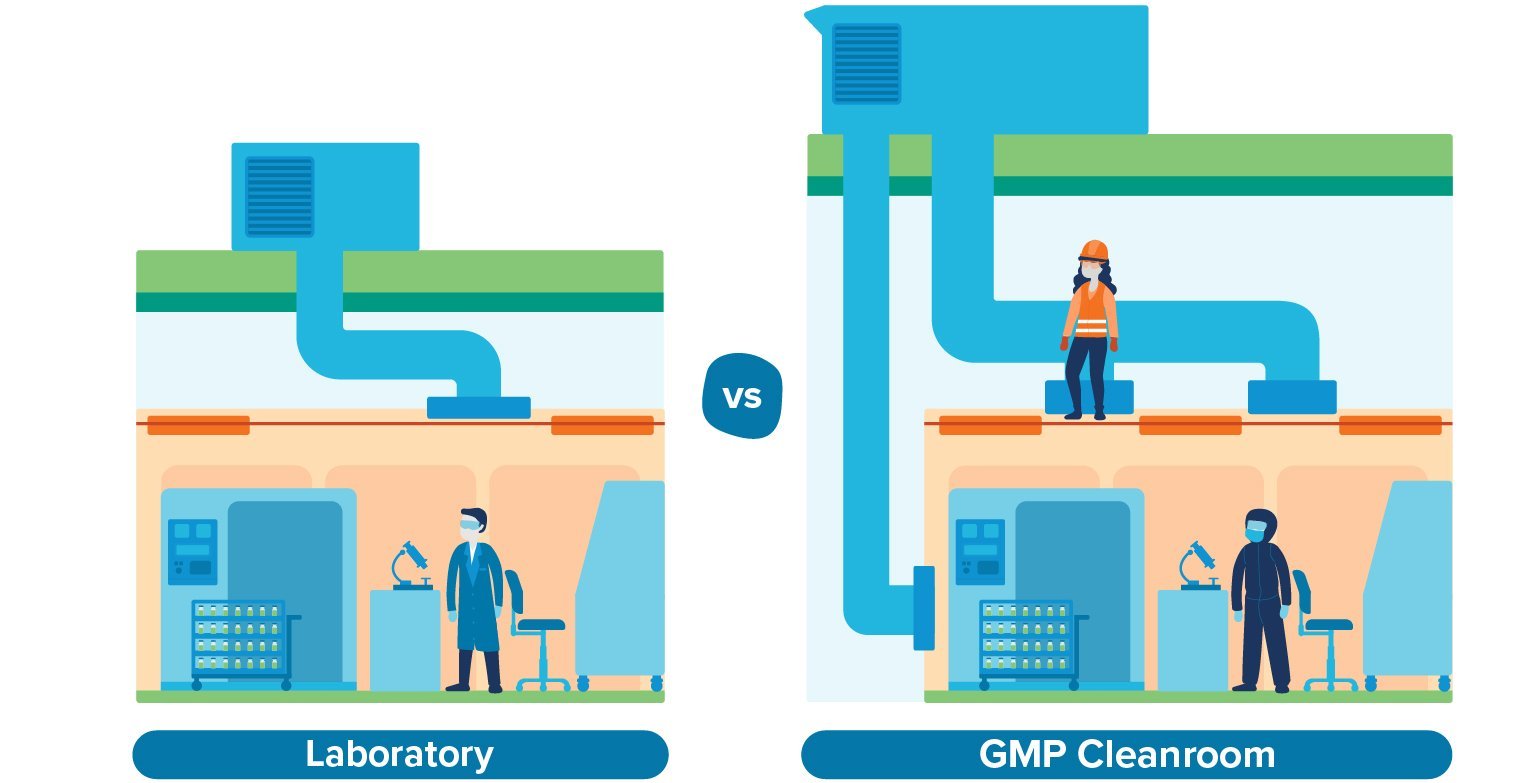
Manufacturers who underestimate these requirements may find themselves removing second-floor slab to accommodate the mechanical systems above first-floor cleanrooms, which was the case for a recent client of ours who came to us with a pre-selected site. As a result, that client is paying for square footage that they’ve had to eliminate—not an enviable situation for any manufacturer.
Shaft Spaces
The COVID-19 pandemic and its role in normalizing work-from-home policies has left commercial landlords with a surplus of multi-level office buildings, many of them in desirable locations and priced to attract even cost-conscious tenants.
For cell and gene therapy manufacturers who want an urban location with access to skilled workers, this situation presents an interesting opportunity. But a multi-level building could introduce new challenges, as well. One key challenge is the issue of connecting air handlers, typically located on the rooftop, with cleanrooms, which are typically on the main floor. In order for air to travel that distance, you’ll need to design a network of vertical shafts, which often means removing leasable square footage at a significant cost.
Future Flexibility
You may pay less to lease a smaller site, but will you find yourself constrained as your throughput requirement grows over time? That question applies not only to cleanroom spaces, but to support spaces as well—gowning areas, quality testing labs, storage and so on. Then there’s the question of accommodating a growing workforce, which means finding more space for amenities, parking, and more.
On the other hand, investing in a core and shell building with room for expansion means paying for more square footage than you’re prepared to use, at least at first. This is where a close alignment between your business plan and your site-agnostic study is especially valuable: You can lean on your early due diligence to guide you through this decision with confidence.
Employee Experience
Getting all of the CGMP details exactly right for your cell and gene therapy facility is key, but to run those CGMP spaces, you need to attract and retain skilled workers. That is an important consideration that should impact your site selection approach.
Inside your building, features that align with LEED and WELL certification criteria can add a lot of value in terms of employee health and well-being. That means looking for sites that will allow for a vibrant, “people-first” design featuring access to outside views, acoustic and thermal comfort, optimized lighting, and more. It also means thinking about non-manufacturing areas, such as attractive collaboration zones, administrative offices, and cafeteria spaces, all of which could help you build and retain a committed workforce.
This principle of attracting skilled workers by selecting and designing sites with employees in mind applies outside of the building, as well. Access to walking trails or attractive scenery could edge you ahead of the competition in terms of talent acquisition. You could also consider the possibility of partnering with local businesses to offer employees discounts or privileged access to the services that are important to them, such as childcare or fitness centers. These intangible benefits may not seem directly related to your site selection process, but they could play a tiebreaking role if you’re caught between two attractive sites, trying to choose the one that’s best for you.
Once you’re aware of these considerations, you can look out for them as you conduct your assessment, bringing you even closer to the facility that’s just right for your project.
Set your sights on the best site
As you approach the site selection process, one of the best ways to set yourself up for success is to work with experts who understand how to synthesize your business plan, your manufacturing needs, and your vision for future expansion into a tailored checklist that’s detailed, flexible, and specific to your unique situation.
Reach out to our team of cell and gene therapy specialists to learn how they can help you approach this process with confidence, and find a site that will serve your project today and far into a successful future.