CRB design-build with Grand River Aseptic Manufacturing picked to manufacture COVID-19 solution
Oct 1, 2020
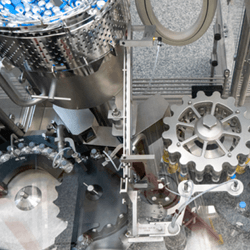
CRB, a market-leading provider of integrated project delivery solutions for vaccine and critical therapy manufacturers, recently completed the design and construction of a large-scale facility for Grand River Aseptic Manufacturing (“GRAM”) that has been selected by the U.S. government to increase domestic pharmaceutical manufacturing for vaccines or therapeutics in the fight against COVID-19.
Built using CRB’s ONEsolution™ project delivery method, GRAM’s new 60,000 square-foot facility in Grand Rapids, Michigan is one of three GRAM pharmaceutical manufacturing facilities. The newest facility was designed to increase GRAM’s large-scale fill and finish capacity with room to expand as demand grows.
CRB’s ONEsolution project team optimized delivery and phasing for GRAM, allowing for fast-tracked procurement, detailed planning, and construction. CRB and GRAM completed the facility on-schedule and on-budget.
“Through close collaboration and enhanced planning with GRAM’s project team, the facility’s operational turn-over, validation and equipment start-up began earlier than otherwise possible, allowing GRAM to begin qualifications and media fills early this year,” said Jesse Adams, project manager for CRB.
As a U.S.-based CDMO, GRAM supports the pharmaceutical supply chain by offering state-of-the-art equipment while delivering high quality products. By providing the capacity to perform advanced aseptic fill and finish services – the last two steps in the manufacturing process for vaccines or therapeutics – GRAM’s facilities will both support the U.S. government and help position the nation’s supply chain to handle future public health emergencies.
“When choosing a design-build partner for our new facility, we needed a team we could rely on to ensure the project would be completed to meet all required regulatory requirements as well as on time and on budget, and we found that with CRB,” said John Wichelt, Vice President Client Pharmaceutical Services at GRAM. “Our new facility gives GRAM the capacity, technology and equipment to support urgent response efforts, starting with U.S.-based manufacturing in the fight against COVID-19.”
About Grand River Aseptic Manufacturing, Inc.
Grand River Aseptic Manufacturing, Inc. (“GRAM”), a leading parenteral contract development and manufacturing organization, delivers customized solutions to meet clients’ fill and finish needs from development through commercialization. With capabilities for biologics as well as controlled substances, GRAM’s expert project managers and modern facilities support pharmaceutical development and cGMP manufacturing, analytical testing, and regulatory filing.
